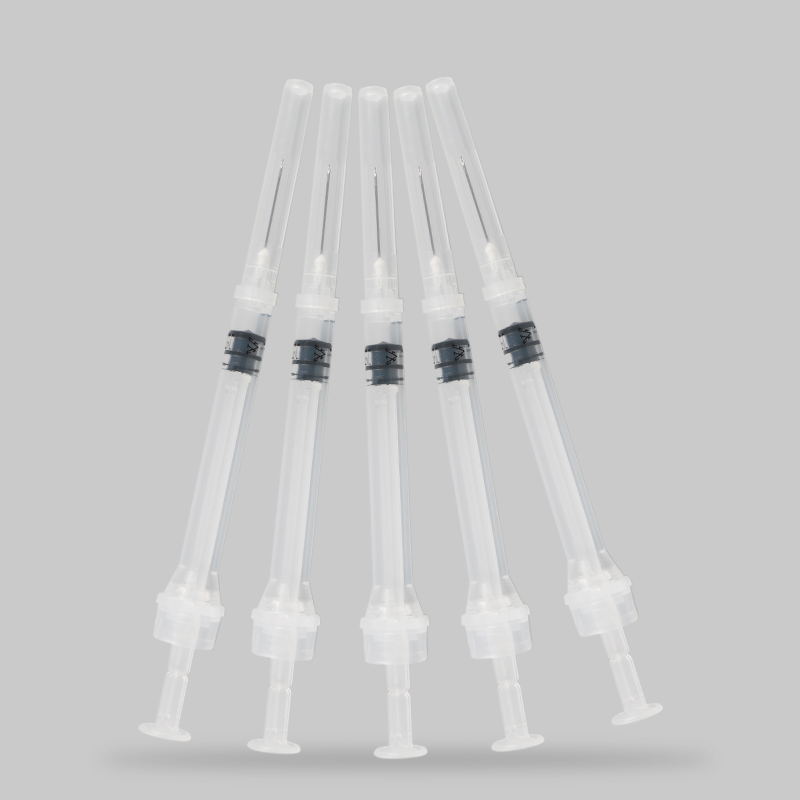
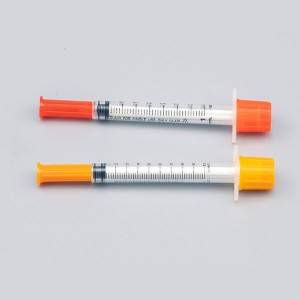
CE/FDA Saogalemu Tu'i tui Eo Fa'amama 0.1ml-5ml Ta'avale Aveesea
CE/FDA Saogalemu Tu'i tui Eo Fa'amama 0.1ml-5ml Ta'avale Aveesea


afifi ma fa'amatalaga
| Tele | Peraimeri | ogatotonu | Pusa | mamafa mama | mamafa mamafa | ||
| Fa'amatalaga(MM) | Fa'amatalaga(MM) | PCS | Fa'amatalaga(MM) | PCS | KG | KG | |
| 1ML | 174*33 | 175*125*140 | 100 | 660*370*450 | 3000 | 9.5 | 15.5 |
| 3ML | 200*36 | 205*135*200 | 100 | 645*420*570 | 2400 | 12 | 18.5 |
| 5ML | 211*39.5 | 213*158*200 | 100 | 660*335*420 | 1200 | 8.5 | 12.5 |
Tulaga lelei & Fetu
Falegaosimea


Fa'aaliga


Tusipasi

Fa'amatalaga a le Kamupani
Jiangxi Sanxin Medtec Co., Ltd., faʻamaumauga faʻatau: 300453, na faavaeina i le 1997. O se atinaʻe faʻatekonolosi a le atunuʻu faʻapitoa i masini faafomaʻi R & D, gaosiga, faʻatau ma auaunaga.A maeʻa le sili atu i le 20 tausaga o le faʻaputuina, o loʻo i ai i le kamupani se vaʻaiga i le lalolagi atoa, mulimulitaʻia taʻiala o le atinaʻeina o le atunuʻu, mulimulitaʻi i manaʻoga faʻapitoa, faʻalagolago i se faiga faʻaleleia lelei ma le matua R & D ma gaosiga lelei, ma ua taʻimua i le alamanuia e pasia. le CE ma le CMD Quality Management system and product certification ma US FDA (510K) fa'atagaina fa'atauga.