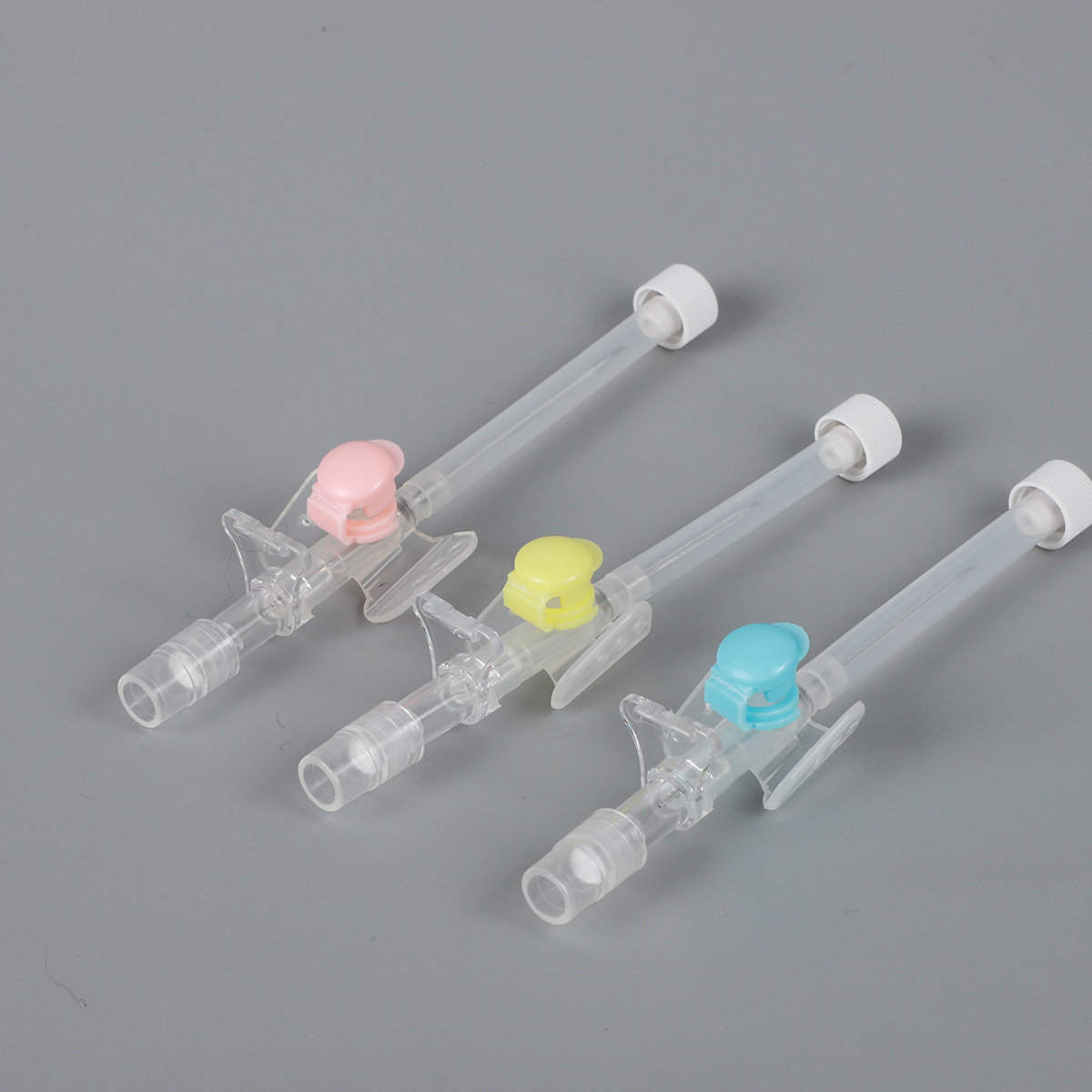
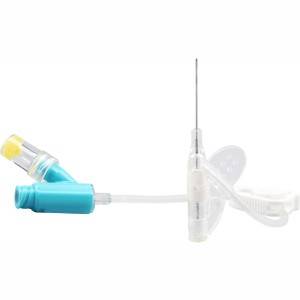
Ata o le Catheter
Tulaga Sa'o-01 IV Catheter
afifi ma fa'amatalaga
| Oloa | Tele | Mea fa'apipi'i | Volume | Lapataiga | Fua (ctns) | mamafa (kgs) | MOQ (seti) |
| Paketi Muamua | Paketi ogatotonu | afifi i fafo | seti /pusa | seti /carton | 20GP | 40HQ | NW | GW |
| Pei-Peni Ituaiga IV Catheter | 14G18G,
20G,22G,
24G,26G | Pulu | Pusa | Pusa | 50 | 800 | 55*28.5*41 | 445 | 1055 | 3.5 | 7.5 | 10000 |
Tulaga lelei & Fetu
1.So'oga pa'u silisi mo le fa'asusu lelei
E i ai se galuega fa'agasolo i luma.A maeʻa le faʻamaʻi, o le a faʻatupuina se tafe lelei pe a liliu ese le seti infusion, e otometi ona tuleia le vai i le IV catheter agai i luma, lea e mafai ona taofia ai le toto mai le toe foʻi mai ma aloese mai le poloka o le catheter.
2. Mea fou, DEHP leai se totogi
O le plasticizer(DEHP)-free polyurethane material o loʻo faʻaaogaina e sili ona lelei le biocompatibility, aloese mai le plasticizer (DEHP) mai le faʻaleagaina o gasegase ma tagata faigaluega fomaʻi.
3. Side pu toto faamalama toe faafoi
O le toe foʻi mai o le toto e mafai ona vaʻaia vave i le taimi sili ona puupuu, lea e mafai ona fesoasoani ia te oe e faʻamasinoina le manuia o le tuʻiina i se taimi vave e mafai ai ma faʻaleleia le tulaga manuia o le tuʻiina.
4. Oomi lima e tasi
O le mamanu faʻatusa mama o loʻo faʻaaogaina i le faʻapipiʻi lima e tasi, o lea e leai se mamafa le lelei o le a faia i le lumen.I le taimi o le pipii, o le a oomi mai se mataua o le vai faʻamaufaʻailoga faʻamau e faʻaleleia ai le aafiaga lelei o le mamafa.
Falegaosimea


Fa'aaliga


Tusipasi

Fa'amatalaga a le Kamupani
Jiangxi Sanxin Medtec Co., Ltd., faʻamaumauga faʻatau: 300453, na faavaeina i le 1997. O se atinaʻe faʻatekonolosi a le atunuʻu faʻapitoa i masini faafomaʻi R & D, gaosiga, faʻatau ma auaunaga.A maeʻa le sili atu i le 20 tausaga o le faʻaputuina, o loʻo i ai i le kamupani se vaʻaiga i le lalolagi atoa, mulimulitaʻia taʻiala o le atinaʻeina o le atunuʻu, mulimulitaʻi i manaʻoga faʻapitoa, faʻalagolago i se faiga faʻaleleia lelei ma le matua R & D ma gaosiga lelei, ma ua taʻimua i le alamanuia e pasia. le CE ma le CMD Quality Management system and product certification ma US FDA (510K) fa'atagaina fa'atauga.


Muamua: Fa'afoma'i saogalemu fa'ama'i fa'amautu tui fa'atama'ia oe lava Sosoo ai: Fa'ama'i fa'ama'i fa'ama'i o tui fa'amau mo le fa'aoga tasi